The daily realities of living with hyperhidrosis, the medical term for excessive and uncontrollable sweating that far surpasses the body’s need for thermoregulation, are often deeply isolating and profoundly disruptive. It is a condition that rarely threatens physical health, yet it can systematically dismantle self-confidence, dictate wardrobe choices, and fundamentally alter professional and social interactions. Unlike the generalized sweating response that occurs from heat or strenuous exercise, this localized, profuse moisture production, frequently concentrating in the axillae (underarms), palms (hands), or soles (feet), occurs seemingly without a clear external trigger. For decades, the therapeutic pathway for patients whose condition proved resistant to standard over-the-counter and prescription-strength antiperspirants was fraught with limited, often high-impact options. These ranged from the inconvenient, time-consuming regimen of iontophoresis—a procedure involving passing a mild electrical current through water—to the drastic and permanent measure of Endoscopic Thoracic Sympathectomy (ETS) surgery, which carries the distinct and often life-altering risk of compensatory sweating, where the body attempts to make up for the blocked sweat in other, often more noticeable, areas. The introduction of Botulinum toxin type A, widely known by the brand name Botox, into the clinical management of severe primary hyperhidrosis, particularly in the axillae, has not merely provided another option but has entirely recalibrated the conversation about effective, non-surgical relief.
The Introduction of Botulinum Toxin Type A Has Not Merely Provided Another Option But Has Entirely Recalibrated the Conversation About Effective, Non-Surgical Relief
The introduction of Botulinum toxin type A, widely known by the brand name Botox, into the clinical management of severe primary hyperhidrosis, particularly in the axillae, has not merely provided another option but has entirely recalibrated the conversation
At the microscopic level, the mechanism by which Botox silences the sweat glands in the localized treatment zone is both elegant and highly specific. Sweating is not merely a passive physiological response; it is an active process initiated by the sympathetic nervous system. The nerves that communicate with the eccrine sweat glands—the most prevalent type of sweat gland—do so by releasing a specific chemical messenger known as acetylcholine. When a nerve signal reaches the sweat gland, acetylcholine is released and binds to receptors on the gland, effectively turning the sweat production “on.” Botulinum toxin, a potent neurotoxin, functions as an interceptor. When small, diluted doses are meticulously injected just beneath the skin’s surface, the toxin is taken up by the peripheral nerve endings at the neuromuscular junction. Here, it cleaves a crucial protein necessary for the transport and release of the acetylcholine-carrying vesicles. By inhibiting the release of this neurotransmitter, the nerve signal is effectively muted, preventing the message from reaching the eccrine gland. The gland itself remains intact and functional, but without its activating signal, it simply ceases the hyper-production of sweat. This temporary and targeted “chemical denervation” offers a period of remarkable dryness, which typically lasts far longer than the cosmetic effects seen when the toxin is used on facial muscles.
When Small, Diluted Doses are Meticulously Injected Just Beneath the Skin’s Surface, the Toxin is Taken Up by the Peripheral Nerve Endings at the Neuromuscular Junction
When small, diluted doses are meticulously injected just beneath the skin’s surface
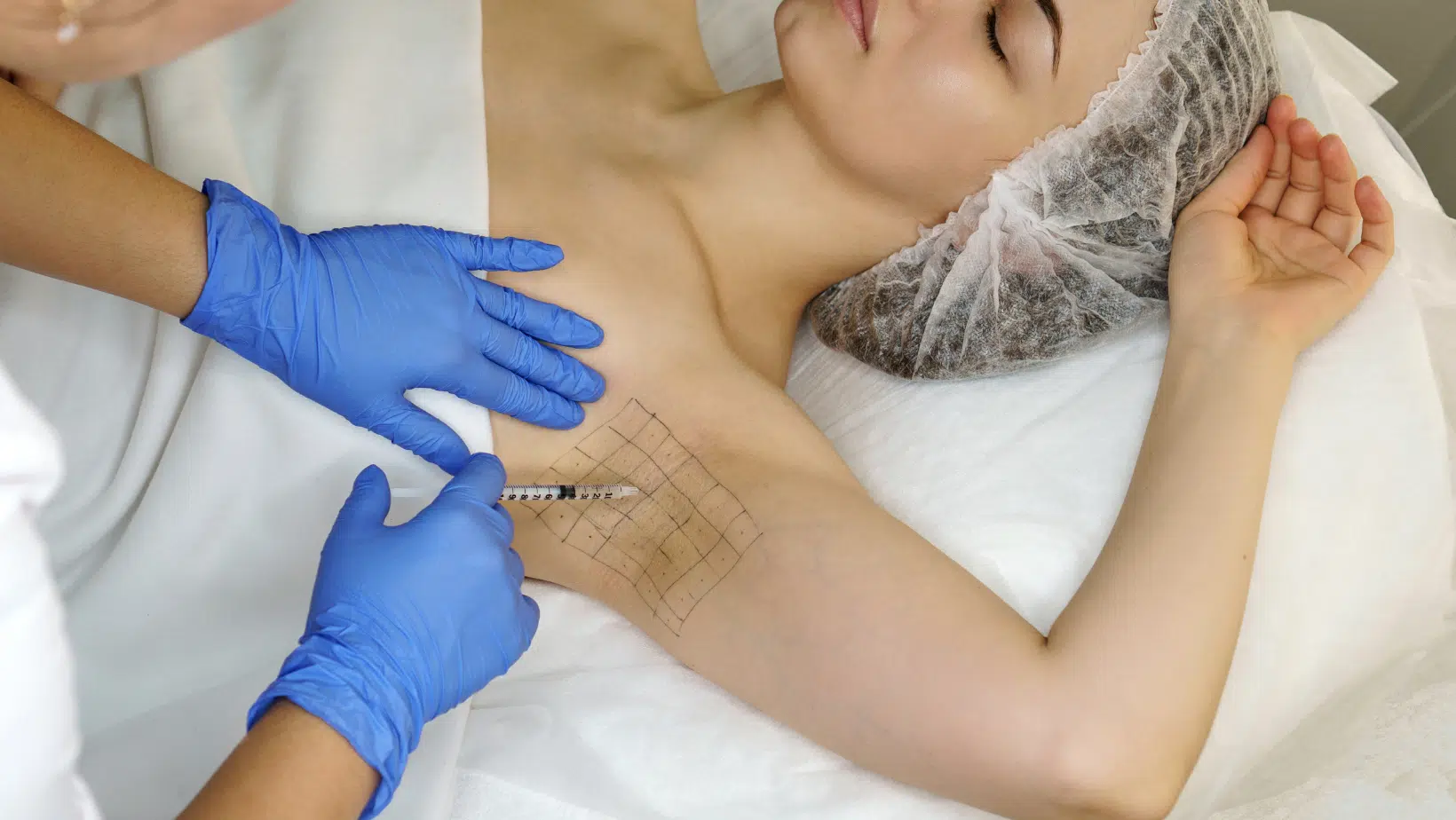
The procedural details surrounding Botox injection for hyperhidrosis are crucial to its success and patient comfort, moving beyond a simple series of jabs. The process usually begins with an optional but frequently recommended Starch-Iodine Test (Minor’s Test). In this simple, low-tech method, an iodine solution is applied to the skin, allowed to dry, and then dusted with starch powder. The presence of sweat, even in small amounts, causes the starch-iodine mixture to turn a distinct dark blue or black color. This visual mapping technique precisely delineates the most active zones of perspiration, providing the clinician with a crucial roadmap for targeted injection placement. The injection itself involves delivering numerous, very small doses of the diluted neurotoxin into the intradermal layer (just under the skin), typically in a precise grid pattern approximately one to two centimeters apart across the entire hyperhidrotic area. For areas like the axillae, where the skin is relatively thin, patients generally tolerate the procedure well, often with just topical anesthetic, icing, or vibration analgesia. However, when treating the palms (palmar hyperhidrosis) or soles (plantar hyperhidrosis), the thicker, tougher skin and the higher density of nerves often necessitate more robust measures, sometimes including a nerve block to manage the heightened sensation, reflecting the procedural complexity involved in these specific body areas.
This Visual Mapping Technique Precisely Delineates the Most Active Zones of Perspiration, Providing the Clinician With a Crucial Roadmap for Targeted Injection Placement
This visual mapping technique precisely delineates the most active zones of perspiration
The experience following treatment is characterized by a gradual, rather than immediate, cessation of excessive sweating. Patients typically report the initial onset of dryness within three to four days following the procedure, but the maximal therapeutic effect is generally not achieved until around two weeks. This latency period is a direct consequence of the toxin’s mechanism of action: it takes time for the neurotoxin to be internalized by the nerve endings and for the protein cleavage process to fully block acetylcholine release across the entire network of treated nerve terminals. Once peak efficacy is reached, the results are remarkably consistent. Clinical trials and real-world data consistently report a reduction in sweating intensity of 80 to 95 percent, an outcome that is genuinely transformative for individuals who have struggled with the constant, visible burden of the condition. This profound relief is not permanent, however, as the body gradually repairs the treated nerve endings, regenerating the necessary proteins over time. The median duration of efficacy for axillary treatment is approximately six to seven months, though individual results can range widely, sometimes lasting as long as ten to twelve months, depending on the patient’s unique metabolism and the severity of their initial condition.
The Median Duration of Efficacy for Axillary Treatment Is Approximately Six to Seven Months, Though Individual Results Can Range Widely
The median duration of efficacy for axillary treatment is approximately six to seven months
While the overall safety profile of Botox for hyperhidrosis is excellent, particularly when administered by an experienced, qualified professional, it is important to acknowledge the spectrum of potential side effects, most of which are mild and self-limiting. The most common issues are localized reactions directly related to the multiple injections, including minor pain, tenderness, swelling, or bruising at the treatment sites. These temporary phenomena resolve quickly within a few days. The most significant non-localized side effect is temporary, mild muscle weakness, particularly when treating the palms. Because the intrinsic muscles of the hand lie close to the target sweat glands, the toxin can occasionally diffuse into these deeper motor nerves, leading to a transient, subtle reduction in grip strength or fine motor control. This effect is consistently reversible and typically fades within two to three weeks as the body processes the toxin. Crucially, the worry that blocking sweat in one area will cause a drastic increase elsewhere—known as compensatory sweating, a serious complication of surgical sympathectomy—is not generally a concern with Botox. The localized nature of the injections and the limited area treated ensures that the body’s overall thermal regulation mechanisms remain fully intact.
The Most Significant Non-Localized Side Effect Is Temporary, Mild Muscle Weakness, Particularly When Treating the Palms
The most significant non-localized side effect is temporary, mild muscle weakness
Beyond the straightforward physiological reduction of sweat production, the true game-changing aspect of this therapy lies in its impact on the patient’s quality of life (QoL). Hyperhidrosis is frequently associated with significant psychosocial morbidity, including anxiety, low self-esteem, avoidance of social or professional situations, and even symptoms of depression. The constant awareness of damp, often stained clothing and the fear of a clammy handshake create a cycle of self-monitoring and emotional distress. By offering a straightforward, non-surgical intervention that delivers near-complete dryness for half a year or more, Botox fundamentally breaks this cycle. Studies measuring QoL metrics, such as the Hyperhidrosis Disease Severity Scale (HDSS) or the Dermatology Life Quality Index (DLQI), consistently demonstrate dramatic improvements, shifting patients from the “intolerable” category to the “tolerable” or even “never noticeable” category. This transformation is not just cosmetic; it facilitates a complete return to normal functioning, restoring the ability to wear any color or fabric, shake hands without apprehension, and engage in social activities without the constant, draining psychological overhead of worrying about visible wetness.
The True Game-Changing Aspect of This Therapy Lies in Its Impact on the Patient’s Quality of Life (QoL)
The true game-changing aspect of this therapy lies in its impact on the patient’s quality of life (QoL)
For those contemplating this treatment, it is important to recognize the current regulatory status, which reflects the strongest evidence base. While Botox is frequently used off-label to treat hyperhidrosis of the hands, feet, face, and scalp, the toxin holds formal FDA approval specifically for the treatment of severe primary axillary hyperhidrosis in patients who have failed to achieve relief with topical agents. The use in other areas, while often highly effective, is a common practice based on clinical experience and secondary literature, but it is not supported by the same primary regulatory designation. Furthermore, the cost of the treatment presents a notable barrier for many patients. Botulinum toxin is a premium-priced biologic product, and a full, effective treatment for bilateral axillae can be costly. While many health insurance providers, recognizing the medical necessity and debilitating nature of severe hyperhidrosis, will cover the treatment after appropriate pre-authorization and documentation of failure with conservative methods, navigating this reimbursement process remains a common administrative hurdle. Patients must be prepared for the financial aspect and understand that this is a recurrent, ongoing treatment, typically needed twice a year.
The Cost of the Treatment Presents a Notable Barrier for Many Patients
The cost of the treatment presents a notable barrier for many patients
The need for a maintenance schedule is a core component of long-term success with Botulinum toxin therapy. Given the temporary nature of the nerve blockade, the patient is required to proactively manage their symptoms by re-engaging with their provider for follow-up treatments. The optimal time for a subsequent injection is not a rigid calendar date but is determined by the re-emergence of symptoms. As the effects begin to fade, the patient typically notices a gradual return of the excessive perspiration, a return to their baseline of a Hyperhidrosis Disease Severity Score (HDSS) of 3 or 4. Clinicians strongly advise against waiting until the symptoms are fully back to their pretreatment severity. Instead, scheduling a follow-up injection just as the symptoms begin to noticeably interfere with daily life again, often at the 6-month mark, ensures a consistent level of control and minimizes the period of discomfort and anxiety. Establishing this regular, preventive treatment cycle is the key to maintaining the dramatic quality-of-life improvements that the initial therapy delivers.
Establishing This Regular, Preventive Treatment Cycle Is the Key to Maintaining the Dramatic Quality-of-Life Improvements That the Initial Therapy Delivers
Establishing this regular, preventive treatment cycle is the key to maintaining the dramatic quality-of-life improvements
Ultimately, the successful deployment of Botulinum toxin for hyperhidrosis rests not just on the neurotoxin itself, but on the careful execution by a skilled practitioner who possesses a precise understanding of the anatomy, proper injection technique, and dosage adjustments required for different body areas. The meticulous, grid-like pattern of micro-injections is designed to ensure maximum coverage of the affected eccrine glands while minimizing the dose to prevent diffusion into surrounding muscles. This high degree of technical precision is particularly non-negotiable for areas like the face or hands, where misplaced injections can lead to issues such as temporary facial asymmetry or hand weakness. For patients considering this route, prioritizing a board-certified dermatologist or plastic surgeon with specific, documented experience in treating hyperhidrosis is the most critical factor, far outweighing any concerns about which specific brand of neurotoxin is used. Their expertise ensures both the effectiveness and the safety of a procedure that, when done correctly, represents one of the most impactful and least invasive treatments available for this debilitating condition.